FORMATION OF TALC
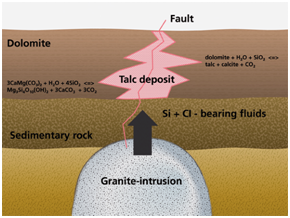
Talc is a metamorphic mineral (1) resulting from the metamorphism of magnesium minerals such as Serpentine, Pyroxene, Amphibole, Olivine, in the presence of Carbon Dioxide and Water. This is known as Talc Carbonation or Steatization and produces a suite of rocks known as Talc Carbonates.
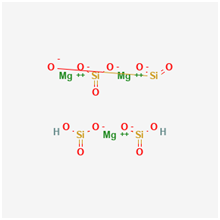
Talc is a mineral of Hydrous Silicate of Magnesium with Chemical Formula of Mg3Si4O10(OH)2 .
1. Talc is primarily formed via hydration and carbonation of serpentine, via the following reaction:
serpentine + carbon dioxide → Talc + magnesite + water
2Mg3Si2O5(OH)4 +
3CO2 → Mg3Si4O10(OH)2 + 3 MgCO3 + 3 H2O
2. Most Talc is formed from the alteration of Dolomite (CaMg(CO3)2 or of Magnesite. (MgO) in the presence of excess dissolved Silica (SiO2). Talc can be formed via a reaction between Dolomite and Silica, which is typical of skarnification of Dolomites via silica-flooding in contact metamorphic aureoles:
dolomite + silica
+ water → Talc + calcite + carbon dioxide
3CaMg(CO3)2 + 4
SiO2 + H2O → Mg3Si4O10(OH)2 + 3 CaCO3 + 3 CO2
3. Serpentine or Quartzite can also form Talc. Talc can be formed from magnesian chlorite and quartz in blueschist and eclogite metamorphism via the following metamorphic reaction:
chlorite + quartz
→ kyanite + Talc + water
Associated Minerals: Dolomite, Magnesite, Quartz, Olivine, Pyroxenes, Serpentine, Amphiboles and Biotite